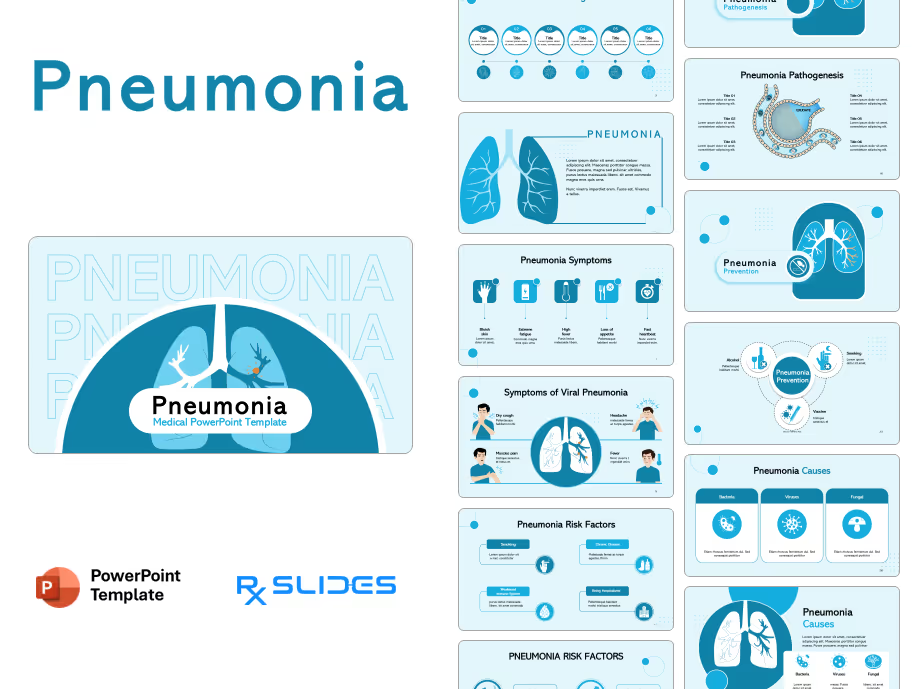
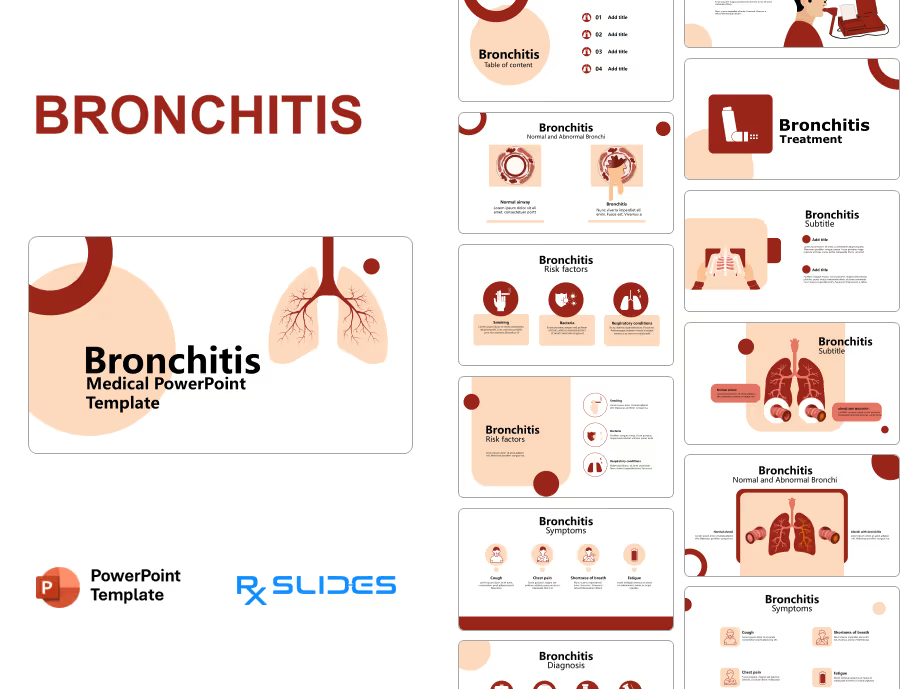
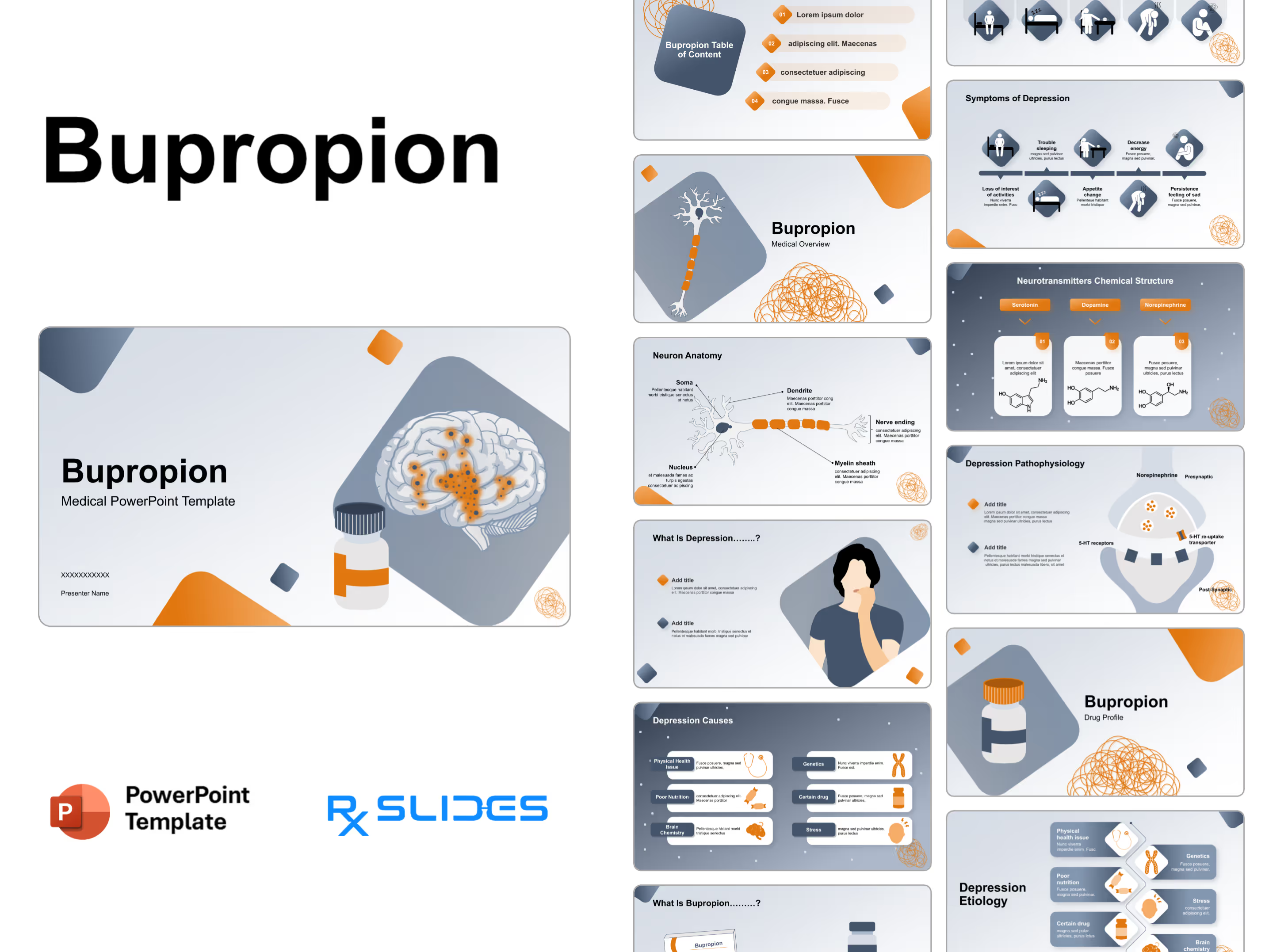
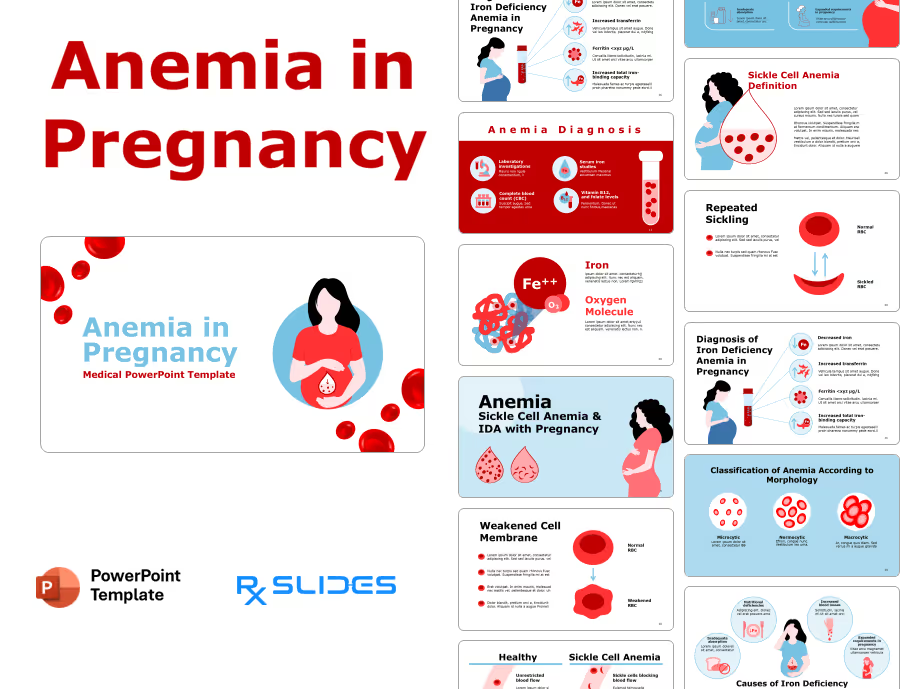
Exenatide PowerPoint Template

Exenatide PPT Template: Medical Animated PowerPoint
- The Exenatide PPT template is an animated medical PowerPoint designed by RxSlides' team of healthcare professionals.
- This dynamic template has animation to simplify the mechanisms of Exenatide, transforming complex medical concepts into clear and engaging visuals.
- Your audience will see Exenatide’s journey through the body, its interaction with receptors and its ultimate impact on blood sugar control; all brought to life through impressive animations.
- Simplify medical processes with step-by-step animated sequences, ensuring your audience understands even the most complex details of Exenatide's action.
Exenatide PPT Template Preview
Content of Exenatide PPT Template
Slide 1 - Exenatide Introduction (Title Slide)
.avif)
- Introduces the presentation on Exenatide using a clean, modern, and professional aesthetic.
- The slide features a prominent graphic of a yellow and teal injection pen.
Slide 2 - Table of Contents (Timeline Navigation)
.avif)
- Outlines the presentation structure.
- The clean, modern design uses a horizontal line with speech bubble callouts.
Slide 3 - Medical Overview (Section Divider)
.avif)
- Section divider to transmit to the medical overview of Exenatide.
- The slide prominently features a stylized illustration of the pancreas.
Slide 4 - Pancreas Anatomy (Diagram)
.avif)
- Details the anatomy of the pancreas.
- The slide features a prominent, well-labeled illustration of the organ, detailing five key parts like the Head, Tail, and Duodenum, making complex information instantly understandable.
Slide 5 - Pancreas Anatomy (Content Layout)
.avif)
- Presents the Pancreas anatomy while simultaneously detailing three key facts or functional points related to the organ.
- The slide features the clear, labeled diagram of the pancreas alongside three color-coded content boxes.
Slide 6 - Pancreas Histology (Cell Detail)
.avif)
- Explains the Pancreas Histology, detailing the specific cells and structures relevant to the function of Exenatide.
- The design features a central, stylized illustration of the Islets of Langerhans surrounded by the Pancreatic Acini, clearly breaking down the microscopic anatomy.
Slide 7 - Islets of Langerhans (Detailed Cell Map)
.avif)
- Explains the crucial components of the Islets of Langerhans, highlighting five different functional cell types.
- The design features a large, central illustration that color-codes and represents the distribution of Alpha, Beta, Delta, and Epsilon cells within the islet structure.
Slide 8 - Insulin Secretion (Mechanism Diagram)
.avif)
- Explains the complete process of Glucose-Dependent Insulin Secretion using clear, step-by-step visuals.
- The slide features a labeled illustration of the Beta cell membrane, detailing the movement of Glucose, ATP, and Calcium through key channels.
- Shows three corresponding summary text boxes that outline the process from glucose entry to insulin release.
Slide 9 - Drug Overview (Section Divider)
.avif)
- Section divider to transmit to the dedicated Drug Overview section of the Exenatide presentation.
- The slide features two prominent, stylized injection pens resting on a subtle ripple effect, immediately reinforcing the visual theme and the drug's delivery method.
Slide 10 - Exenatide Chemical Structure (Comparison)
.avif)
- Contrasts the chemical makeup of Exenatide with the natural hormone GLP-1.
- The slide features two primary, color-coded diagrams that represent the peptide chains, allowing for a clear and immediate visual comparison of their structures.
Slide 11 - Exenatide Chemical Structure (Evolution)
.avif)
- Shows how the drug Exenatide is chemically derived or evolved from the natural hormone GLP-1.
- The slide features side-by-side diagrams of the two chemical structures, linked by an arrow graphic.
Slide 12 - Exenatide Chemical Structure (Visual Contrast)
.avif)
- Shows how the drug Exenatide is chemically different from the natural hormone GLP-1 in a side-by-side view.
- The slide features two colored diagrams of the peptide structures linked by an arrow, making the minor but crucial chemical changes instantly visible.
Slide 13 - Exenatide Chemical Structure (Feature Detail)
.avif)
- Focuses on the Exenatide chemical structure while providing four different points of content about its features or function.
- The design features a large, central, color-coded diagram of the entire peptide chain, visually dominating the slide to ensure the structure is the main focus.
Slide 14 - Exenatide Classification (Category Introduction)
.png)
- Introduces the drug classification, highlighting that Exenatide belongs to the category of Incretin mimetics.
- The slide features a prominent text section for the classification details alongside a 3D-style visual of both the GLP-1 and Exenatide chemical structures for context.
Slide 15 - Exenatide Classification (Comparative Content)
.png)
- Details two key aspects of Exenatide classification.
- The design features side-by-side text boxes flanking the chemical structure diagrams of GLP-1 and Exenatide.
Slide 16 - Diabetic Drugs Self-Regulation (Mechanism Comparison)
.png)
- Highlights the critical difference between the self-regulating activity of Exenatide and older diabetic drugs.
- The design uses a central "Glucose level" meter.
Slide 17 - Exenatide Dosage Forms (Presentation)
.png)
- Presents the different Exenatide Dosage Forms.
- The design features two distinct, colored pen injector graphics and corresponding text boxes that detail key differences, such as dosage amounts (5 mcg vs. 10 mcg) and total volume.
Slide 18 - Exenatide Dosage (5 mcg Feature Summary)
.png)
- Summarizes the key features and indications of the 5 mcg Exenatide dosage using an engaging, central package visual.
- The design places the drug packaging graphic at the center, with three icon-driven content boxes floating above it to detail key usage points.
Slide 19 - Exenatide Dose (10 mcg Workflow)
.png)
- Details four sequential points related to the 10 mcg Exenatide dose.
- The design features the drug packaging at the center, surrounded by a circular flow graphic connecting four numbered, yellow content areas.
Slide 20 - Pharmacokinetics (Section Divider)
.png)
- Section divider to transmit to Pharmacokinetics section.
- The slide features a simple, three-step illustration showing drug molecules (yellow dots) being released from a carrier structure (teal hexagons).
Slide 21 - Exenatide Volume of Distribution (Compartments)
.png)
- Explains the volume of distribution for Exenatide.
- The design features a visual diagram that breaks down the 28.3 L total volume into Plasma 4 L, Interstitium 10L, and Cells 28 L.
Slide 22 - Exenatide Clearance (Excretion Rate)
.png)
- Communicates the rate and method of Exenatide clearance, a critical pharmacokinetic parameter.
- The design features a large graphic of a kidney next to an orange ring displaying the 9.1 L/hour clearance value, immediately establishing the route of drug elimination.
Slide 23 - Exenatide Pharmacokinetics (Three-Step Process)
.png)
- Breaks down a key three-step process in Exenatide's drug action or release.
- The design features three progression graphics showing drug molecules being released from a carrier structure.
Slide 24 - Exenatide Pharmacokinetics (Step-by-Step Flow)
.png)
- Details the three-step flow of Exenatide's pharmacokinetics, ensuring maximum clarity for the audience.
- The design features three numbered cards (01, 02, 03), each containing a corresponding graphic illustrating the sequential process of drug release or action.
Slide 25 - Exenatide Half-Life (Key Timing)
.png)
- Emphasizes the Exenatide half-life.
- The design features a large, clock-like graphic prominently displaying the 2.4 hours half-life value.
- Shows four surrounding text areas and an injection pen graphic.
Slide 26 - Mechanism of Action (Section Divider)
.png)
- Section divider to transmit to the Mechanism of action section.
- The slide features three brightly colored, interlocking gear graphics.
Slide 27 - Exenatide Mechanism of Action (Cellular Detail)
.png)
- Illustrates the Exenatide mechanism of action.
- The design features two complementary diagrams: one showing Exenatide's presence alongside glucose in a vessel, and the other detailing the cellular cascade involving the GLP-1 receptor and Adenylyl cyclase.
Slide 28 - Indications (Section Divider)
.png)
- Section divider to transmit into the clinical application, focusing on the Indications for Exenatide.
- The slide features a prominent graphic of a yellow prescription pad with a large 'RX' symbol.
Slide 29 - Exenatide Indications (Diabetes Focus)
.png)
- Emphasizes the primary indication for the drug, highlighting the use of Exenatide for Diabetes management.
- The design features a prominent, focused illustration of hands using a blood glucose meter.
Slide 30 - Exenatide Indications (Glucose Control)
.png)
- Shows the indication of Exenatide for Diabetes treatment by focusing on the goal of blood glucose control.
- The design features a large graphic of a blood glucose meter displaying a key reading next to a simplified red blood droplet.
Slide 31 - Exenatide Indications (Content Slide)
.png)
- Present four key indications or aspects of Exenatide and Diabetes treatment to your audience.
- The design instantly attracts your audience attention with a central blood glucose monitor graphic and a prominent red blood drop, creating a strong visual focus on the medical topic.
Slide 32 - Side Effects (Section Divider)
.png)
- Section divider to transmit into the Exenatide Side effects and important safety considerations.
- The slide features a large, central graphic of a blood glucose meter displaying a prominent warning sign (exclamation point).
Slide 33 - Exenatide Side Effects (Key Symptoms)
.png)
- Presents the six most common Exenatide side effects, grouped logically by system (neurological and gastrointestinal).
- The design uses six numbered text boxes, each paired with a relevant icon (like a dizzy head or a stomach).
Slide 34 - Exenatide Side Effects (Visual List)
.png)
- Presents six key Exenatide side effects in vertical column format.
- The design utilizes six distinct icons (such as figures representing nausea, vomiting, and headache) placed above the corresponding text for instant symptom recognition.
Slide 35 - Clinical Studies (Section Divider)
.png)
- Section divider to transmit to Clinical studies and research findings related to Exenatide.
- The slide features a prominent graphic of a clipboard with a checklist.
Slide 36 - Clinical Study Introduction (Context Setting)
.png)
- Introduces the Exenatide Clinical Study section.
- The design uses two supporting visuals: a simplified injection pen graphic and a pancreas illustration.
Slide 37 - Clinical Study Methods (Data Split)
.png)
- Illustrates the Methods used in the Exenatide clinical study by comparing two distinct approaches.
- The design features two percentage graphics (25% and 75%).
Slide 38 - Clinical Study Methods (Research Phases)
.png)
- Presents the five phases of the Exenatide clinical study.
- The design organizes the phases (one through five) in a flow chart pattern around a central magnifying glass.
Slide 39 - Clinical Study Results (Bar Chart Data)
.png)
- Presents the Results of the Exenatide clinical study.
- The design uses a bar chart to highlight a clear contrast (75% versus 25%), immediately communicating the significant positive findings of the treatment.
- Supported by icons for drug administration and test tubes.
Slide 40 - Clinical Study Conclusions (Wrap-up)
.png)
- Summarizes the key "Take home message" from the entire clinical study and smoothly transitions to the Q&A segment.
- The design uses two distinct speech bubble graphics—one with a checkmark for conclusions and one with a question mark.
Slide 41 - Clinical Study Conclusions (Final Message)
.png)
- Delivers the "Take home message" from the clinical study and clearly invites Questions.
- The design uses large, colored pill-shaped buttons with checkmark and question mark icons.
Slide 42 - Contraindications (Safety Section Divider)
.png)
- Section divider to transmit to Exenatide Contraindications and safety constraints.
- The slide features a prominent visual of an injection pen overlayed with a large red 'Stop' symbol.
Slide 43 - Exenatide Contraindications (Safety Breakdown)
.png)
- Details the four main Exenatide contraindications that restrict the drug's use.
- The design uses a quarter-circle layout to separate and highlight each condition such as Diabetes Type I and severe Kidney impairment.
- With clear, icon-supported categories including Hypersensitivity and Thrombocytopenia.
Slide 44 - Exenatide Cautions (Disease Risk)
.png)
- Details three crucial Cautions when using Exenatide.
- The design uses three colored boxes linked to corresponding organ icons (Pancreas, Kidney, and Gastric).
Slide 45 - Monitoring Parameters (Kidney Focus)
.png)
- Communicates the essential Monitoring Parameters for patients taking Exenatide, focusing on long-term safety.
- The design features a large, dominant illustration of the kidney.
Slide 46 - Exenatide Monitoring (Schedule & Timing)
.png)
- Presents the recommended Exenatide monitoring schedule and key timing parameters for follow-up appointments.
- The design features a large, central calendar graphic, visually representing the necessary periodic monitoring, with four text boxes linked to specific days (yellow and orange squares).
Slide 47 - Drug Interactions (Final Section Divider)
.png)
- Section divider to transmit to Exenatide Drug interactions with other medications.
- The slide features two prominent visuals: an injection pen and a traditional drug vial.
Slide 48 - Drug Interactions (Cidofovir Warning)
.png)
- Details a specific, Contraindicated Additive effect between Exenatide and the antiviral drug Cidofovir.
- The design clearly uses the formula of Exenatide + Cidofovir and highlights the severe risks of the combination, including myelosuppression and nephrotoxicity.
Slide 49 - Drug Interactions (Cidofovir Risk Summary)
.png)
- Summarizes the key risks of the Contraindicated Additive effect between Exenatide and Cidofovir.
- The design visually places the Exenatide pen flowing into the Cidofovir vial and highlights the severe risks, including increased nephrotoxicity and myelosuppression.
Slide 50 - Drug Interactions (Potassium Warning)
.png)
- Details a specific, Contraindicated Additive effect between Exenatide and Potassium supplements.
- The design uses a visual formula (Exenatide + Potassium) and clearly outlines the risks, including gastrointestinal ulceration and transit delay.
- With warning icons illustrating gut lining damage and blocked transit.
Slide 51 - Drug Interactions (Potassium Risk Summary)
.avif)
- Summarizes the key risks of the Contraindicated Additive effect between Exenatide and Potassium supplements.
- The design visually depicts the Exenatide pen connected to the Potassium tablet container.
- With warning icons illustrating gut damage and gastrointestinal transit delay.
Slide 52 - Thank You (Closing Slide)
.avif)
- Concludes the presentation with a clear "Thank you" message.
- The design features a large, stylized injection pen graphic, subtly reinforcing the presentation's core topic even in the final moments.
Features of the Template
- 100% editable PowerPoint template.
- Editable colors, you can change according to your presentation style and company branding guidelines.